Sinou Natalia 1,2, Sinou Nikoleta 1,2, Daskalopoulou Dimitra1, Chrysikos Dimosthenis1, Troupis Theodoros1, Filippou Dimitrios1,2
1Dept. of Anatomy, Medical School, National and Kapodistrian University of Athens, Greece
2 Research and Education Institute of Biomedical Science
Correspondence Address: Natalia Sinou, MD, Email: sinou.natalia@gmail.com
doi: 10.5281/zenodo.17108054.
Circumportal pancreas is an uncommon congenital defect where pancreatic tissue fully surrounds the portal vein and/or the superior mesenteric vein. Typically, it is discovered incidentally during imaging studies or during regional surgeries conducted for different issues. It can sometimes be overlooked, leading to intra- and postoperative complications, such as pancreatic fistulas, infections, and hemorrhage.
This study aims to increase awareness of this rare anatomical variation to avert serious repercussions during pancreatic procedures. Thorough research was performed using the PubMed database with the search terms: “circumportal” and “pancreas”.
It is crucial to recognize circumportal pancreas before surgery to understand ductal anatomy and avoid potentially life-threatening complications. MRI and CT scans are essential for detecting this condition along with any related vascular abnormalities.
Keywords: “Circumportal”, “Pancreas”
Introduction
Circumportal pancreas (CirP) is a rare and often overlooked congenital pancreatic anomaly. In this condition, pancreatic tissue completely surrounds the portal vein (PV) and/or the superior mesenteric vein (SMV). Literature suggests that the prevalence of this abnormality varies between 1.14% and 2.5%, highlighting its rarity (1,2). There is no evidence of sexual predominance for this anomaly, and it is considered less common than other pancreatic variations like annular pancreas or pancreas divisum (3). Various terms exist to describe the same condition, including retroportal pancreas, portal annular pancreas, and complete encasement of the portal vein by pancreatic tissue (4). The first documented case of CirP appeared in 1987 by Sagiura et al., who inadvertently identified a circumportal pancreas encompassing the superior mesenteric vein during pancreatic surgery. Since that time, numerous publications have emerged, predominantly chronicling individual case reports (1).
Typically, CirP is asymptomatic and is frequently discovered incidentally during pancreatic surgery
or imaging conducted for unrelated issues (5). This report aims to raise awareness of this anatomical variation to prevent serious intra-operative and post-operative complications, such as infection, hemorrhage, and pancreatic fistula.
Materials and Methods
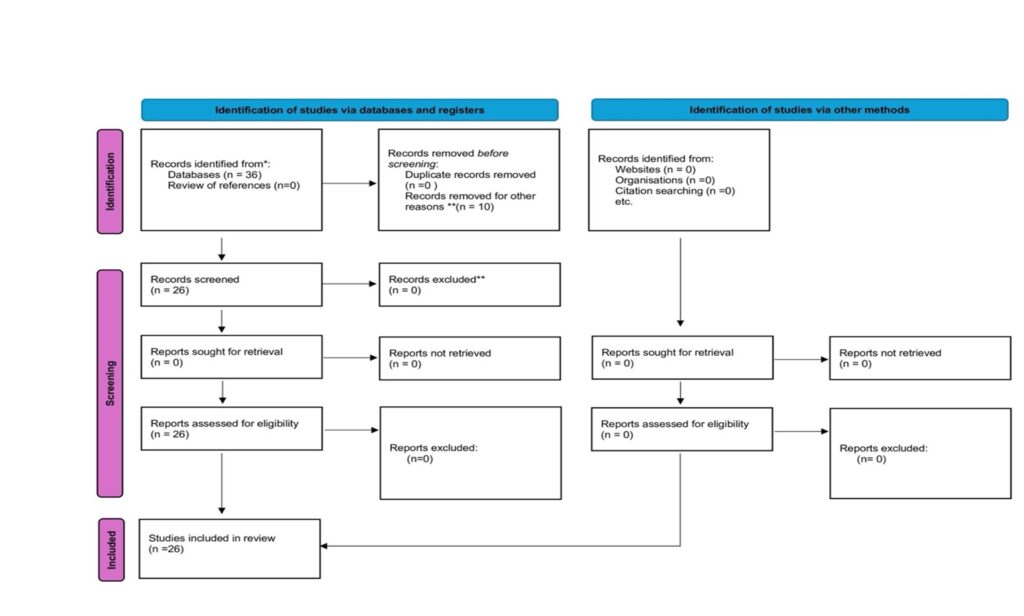
A comprehensive investigation was performed using the published literature obtained from PubMed with the keywords: “circumported” and “pancreas.” Data extraction was carried out through a standardized data collection form based on the specified keywords. The research adhered to the PRISMA 2020 flow diagram for new systematic reviews, which included searches of various databases, registers, and other relevant resources. Pertaining to PRISMA guidelines, the initial records identified through the PubMed search amounted to 36. All 36 full-text articles were evaluated for eligibility, with 10 records excluded due to non-relevant titles and abstracts. Ultimately, none of the articles assessed for eligibility were excluded, and no additional filters were applied. In conclusion, 26 references that met the specified criteria were utilized in this study.
Figure 1. PRISMA 2020 flow diagram for new systematic reviews
|
Results
The vast majority of the studies demonstrated that there is no difference between sexes in the prevalence of circumported pancreas. Moreover, no background or environmental factors were not reported in the development of this rare abnormality. There are no data indicating reasons causing this embryological abnormal fusion. Finally, there are no symptoms in most of the cases and the discovering of this variance is incidentally in investigation of the area for other reasons.
Discussion
The typical pancreas originates from two separate anlagen within the embryonic foregut, specifically an endodermal tube that develops into a ventral and dorsal pancreatic bud during the fourth week of embryonic development (6). The larger dorsal
bud gives rise to the dorsal pancreas (body and tail) as well as the anterior part of the pancreatic head, while the ventral pancreatic bud forms both the uncinate process and the posterior segment of the pancreatic head, collectively making up the ventral pancreas (7). The uncinate process, anteromedial in position and located behind the portal vein and/or the superior mesenteric vein, typically does not merge with the pancreatic body.
Each component drains secretions into the foregut via their respective ducts, namely the ventral and dorsal ducts. The ventral and dorsal buds arise on opposite sides of the foregut. It has been estimated that during the seventh week of gestation, the ventral bud rotates counterclockwise toward the dorsal pancreatic bud to fuse. However, recent work by Kin et al (5) suggests that the significant growth on the left side of the primitive duodenum promotes the passive relocation of the ventral pancreas posterior to the duodenum and its eventual fusion with the dorsal bud. Thus, the formation of the mature gland occurs through the merging of both the ductal systems and parenchyma of the ventral and dorsal buds around the seventh week of fetogenesis (8). In the developed pancreas, the ventral duct serves as the primary channel for drainage into the major duodenal papilla, while the dorsal duct either fully involutes at the minor papilla (in 30% of cases) or partially involutes, contributing some drainage to the minor papilla (in 60% of individuals).
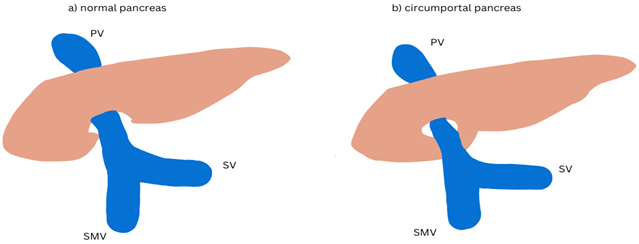
This intricate and differential movement and fusion of pancreatic buds pre-dispose the gland to various anomalies, including pancreas divisum, annular pancreas, and circumportal pancreas (9). Unlike the typical annular pancreas, in which the pancreatic parenchyma surrounds the descending duodenum, circumportal or portal annular pancreas represents a variant where the uncinate process encircles the portal vein and/or the superior mesenteric vein (10). Therefore, it is suggested that an aberrant fusion of the ventral and dorsal buds (occurring cranially to and left of the portal/superior mesenteric vein) results in a ring of pancreatic tissue encircling these vessels.
Joseph et al categorize circumportal pancreas into three types based on the primary pancreatic duct’s (MPD) pathway. Type I features the ventral pancreatic bud merged with the body of the pancreas, associated with a retroportal MPD. Type II includes the same characteristics as Type I but presents with pancreas divisum. Finally, Type III describes an encasing uncinate process with an anterolateral MPD route (11,12). Furthermore, Karasaki et al propose subdivisions based on the fusion relationship between the uncinate process and pancreatic body in relation to the splenic vein: Type A (suprasplenic), Type B (infrasplenic), and Type C (mixed). Type IIIa is the most frequently encountered subtype (44.4-82%) followed by Type Ia (5-27.8%) (13). However, these classifications do not encompass all reported variations of circumportal pancreas to date (14).
Circumportal pancreas typically does not exhibit distinct clinical symptoms. Most cases are incidentally identified during imaging conducted for other reasons or surgical processes (15). It may be misinterpreted as a mass in the pancreatic head, a tumoral mass surrounding the portal vein, or a mass situated posterior to it (16). Notably, 52.9% of intraoperatively identified circumportal pancreas cases were overlooked in preoperative imaging (17). MRI is considered superior to CT as it can visualize the accessory pancreatic duct, making it preferable for patients needing pancreatic surgery. A CT scan or MRI is generally adequate for diagnosing circumportal pancreas, requiring the imaging of the uncinate process adjacent to the pancreatic body across two or more contiguous sections. While contrast-enhanced CT offers a clearer assessment, it is not strictly necessary (18). Additionally, a retroportal MPD path is better delineated through contrast-enhanced CT imaging (including arterial and portal phases) (19). In MRI, fat-suppressed T2-weighted and contrast-enhanced fat-suppressed T1-weighted images effectively outline circumportal pancreas and the retroportal main pancreatic duct. Employing multiplanar reformats derived from thin CT slices is essential for improved depiction accuracy of the anomaly, while careful consideration must be given to avoid confusing peripancreatic lymphadenopathy or a distended caudate lobe of the liver with circumportal pancreas.
Moreover, arterial phase CT may assist in assessing hepatic artery anatomy, as Ishigami et al noted that 25% of individuals with circumportal pancreas displayed atypical arterial anatomy, including a replaced right hepatic artery (from the superior mesenteric artery), a replaced left hepatic artery (from the left gastric artery), and an abnormal course of the common hepatic artery traversing pancreatic tissue.
Circumportal pancreas cases often present with accompanying vascular variations, such as unusual extensions of the celiac artery or common/right hepatic artery. These variations can considerably influence intraoperative outcomes and lead to multiple complications. Common variations include the transition of common hepatic artery through pancreatic parenchyma and the right hepatic artery’s replacement by the superior mesenteric artery (SMA)(19).
Figure 2. Schematic illustration depicting a) normal pancreas b) circumportal pancreas and portal vein (PV) splenic vein (SV) and superior mesenteric vein (SMV)
|
Inadequate preoperative detection of this abnormality may significantly impact surgical procedures (20). The retroportal course of pancreatic tissue necessitates additional resection posterior to the portal vein (PV) or superior mesenteric vein (SMV) during pancreatic head resections, expanding the resection area (21,22). Consequently, pancreatojejunal reconstruction becomes complex, requiring a partial dorsal placement relative to the PV or SMV (23). This added complexity notably amplifies the risk of postoperative pancreatic fistula occurrence, with a reported incidence of 46.7%. Additionally, anatomical variations of the common or right hepatic artery can complicate surgical preparation, particularly in instances of suprasplenic vein involvement with atypical arterial pathways arising from the celiac trunk. The most challenging type of circumportal pancreas may be the mixed type, where both suprasplenic and infrasplenic vessel encasing necessitates three divisions (24).
In summary, it is crucial for surgeons to recognize circumportal pancreas, as it may elevate the risk of pancreatic leakage, particularly when accompanied by a retroportal main pancreatic duct (25).
Conclusion
It is extremely important for pancreatic surgeons to thoroughly examine and identify CirP in preoperative imaging to understand the ductal anatomy and connections (26). Diagnosing this condition can be difficult, yet it is essential to prevent intra- and postoperative risks that could endanger patients’ lives. An accurate preoperative diagnostic assessment includes contrast-enhanced CT scans and MRI (T1-weighted and T2-weighted) imaging. Because CirP can be challenging to detect via imaging, vascular variants like a right hepatic artery originating from the SMA may provide helpful clues regarding the presence of CirP.
Abbreviations
CirP: Circumported Pancreas, PV: Portal Vein, SMV: Superior Mesenteric Vein, SMA: Superior Mesenteric Artery, CT: Computed Tomography, MRI: Magnetic Resonance Imaging
References:
- Luu AM, Braumann C, Herzog T, Janot M, Uhl W, Chromik AM. Circumportal Pancreas-a Must Know Pancreatic Anomaly for the Pancreatic Surgeon. J Gastrointest Surg. 2017 Feb;21(2):344-351. doi: 10.1007/s11605-016-3315-8
- Kabir T, Xuan ZTZ, Chung AYF. Circumportal pancreas: A report of two cases. Ann Hepatobiliary Pancreat Surg. 2019 Aug;23(3):300-304. doi: 10.14701/ahbps.2019.23.3.300.
- Kin T, Shapiro AM. Circumportal pancreas and islet isolation. Surgery. 2009 Jul;146(1):126-7. doi: 10.1016/j.surg.2008.04.017.
- Connelly TM, Sakala M, Tappouni R. Circumportal pancreas: a review of the literature and image findings. Surg Radiol Anat. 2015 Jul;37(5):431-7. doi: 10.1007/s00276-015-1436-5.
- Arora A, Velayutham P, Rajesh S, Patidar Y, Mukund A, Bharathy KG. Circumportal pancreas: a clinicoradiological and embryological review. Surg Radiol Anat. 2014 May;36(4):311-9. doi: 10.1007/s00276-013-1189-y.
- Kin T, Shapiro J. Partial dorsal agenesis accompanied with circumportal pancreas in a donor for islet transplantation. 2010 May-Jun;2(3):146-8. doi: 10.4161/isl.2.3.11715.
- Ishigami K, Tajima T, Nishie A, Asayama Y, Kakihara D, Nakayama T, Shirabe K, Taketomi A, Nakamura M, Takahata S, Ito T, Honda H. The prevalence of circumportal pancreas as shown by multidetector-row computed tomography. Insights Imaging. 2011 Aug;2(4):409-414. doi: 10.1007/s13244-011-0092-5.
- Kiuchi R, Mizuno T, Okamura Y, Sugiura T, Kanemoto H, Uesaka K. Circumportal pancreas – a hazardous anomaly in pancreatic surgery. HPB (Oxford). 2018 May;20(5):385-391. doi: 10.1016/j.hpb.2017.10.009.
- Shonaka T, Inagaki M, Akabane H, Yanagida N, Shomura H, Kudo T, Orimo T, Oikawa F, Aiyama T, Yanagawa N, Oikawa K, Nakano S. Pancreatoduodenectomy for circumportal pancreas accompanying the retroportal pancreatic duct: a case report and review of the literature. Clin J Gastroenterol. 2012 Oct;5(5):332-5. doi: 10.1007/s12328-012-0326-1.
- Kim R, Marfil-Garza BA, Shapiro AMJ, Kin T. Circumportal pancreas accompanied with pancreas divisum in a deceased donor for islet transplantation. Surg Radiol Anat. 2018 Nov;40(11):1323-1325. doi: 10.1007/s00276-018-2072-7.
- Imamura H, Adachi T, Yamashita M, Kinoshita A, Hamada T, Matsushima H, Hara T, Soyama A, Kobayashi K, Kanetaka K, Eguchi S. Minimally invasive pancreaticoduodenectomy for circumportal pancreas: literature review and report of two type IIIA cases. Surg Case Rep. 2024 Jul 29;10(1):175. doi: 10.1186/s40792-024-01979-7.
- Parray AM, Nadkarni S, Chaudhari V, Shrikhande SV, Bhandare MS. Pancreaticoduodenectomy in the Portal Annular Pancreas-Mesopancreas Triangle Approach (with Video). Ann Surg Oncol. 2023 Sep;30(9):5758-5760. doi: 10.1245/s10434-023-13782-z.
- Tappouni R, Perumpillichira J, Sekala M, Hosseinzadeh K, Clark C, Leyendecker J. Circumportal pancreas: imaging findings in 40 patients. Abdom Imaging. 2015 Mar;40(3):521-30. doi: 10.1007/s00261-014-0242-6.
- Ohtsuka T, Mori Y, Ishigami K, Fujimoto T, Miyasaka Y, Nakata K, Ohuchida K, Nagai E, Oda Y, Shimizu S, Nakamura M. Clinical significance of circumportal pancreas, a rare congenital anomaly, in pancreatectomy. Am J Surg. 2017 Aug;214(2):267-272. doi: 10.1016/j.amjsurg.2016.11.018.
- Hashimoto Y, Ross AS, Traverso LW. Circumportal pancreas with retroportal main pancreatic duct. 2009 Aug;38(6):713-5. doi: 10.1097/MPA.0b013e3181a910ca.
- Ahad Aziz Qureshi PA, Yaseen MT, Tariq TA, Niazi IK. The uncommon loop: Circumportal annular pancreas. J Pak Med Assoc. 2019 Dec;69(12):1927.
- Yilmaz E, Celik A. Circumportal pancreas: prevalence, subtypes and vascular variations of 55 patients. Surg Radiol Anat. 2018 Apr;40(4):407-413. doi: 10.1007/s00276-018-1975-7.
- Gonoi W, Akahane M, Akai H, Hagiwara K, Kiryu S, Hayashi N, Ohtomo K. Retroportal main pancreatic duct with circumportal pancreas: radiographic visualization. Clin Imaging. 2011 Nov-Dec;35(6):442-6. doi: 10.1016/j.clinimag.2011.01.002.
- Leyendecker JR, Baginski SG. Complete pancreatic encasement of the portal vein (circumportal pancreas): imaging findings and implications of a rare pancreatic anomaly. J Comput Assist Tomogr. 2008 Jan-Feb;32(1):61-4. doi: 10.1097/rct.0b013e3180557448.
- Do JE, Goh SK, Saxon S, Thomson JE. Pancreatic neuroendocrine tumour resection in circumportal pancreas: a rare anatomical anomaly with important surgical implications. BMJ Case Rep. 2024 Mar 19;17(3):e257013. doi: 10.1136/bcr-2023-257013.
- Kawamoto H, Fujikawa T, Tanaka A. Successful resection of pancreatic head cancer in a patient with circumportal pancreas: a case report with technical consideration. Innov Surg Sci. 2017 Feb 28;2(1):33-37. doi: 10.1515/iss-2017-0003.
- Kulemann B, Hoeppner J. Extended pancreatic head resection for pancreatic cancer in the presence of a circumportal pancreas. ANZ J Surg. 2019 Jan;89(1-2):124-125. doi: 10.1111/ans.14015.
- Nagai K, Masui T, Anazawa T, Hatano E. Laparoscopic pancreatoduodenectomy for a metastatic tumor in a portal annular pancreas. Surg Oncol. 2022 Jun;42:101772. doi: 10.1016/j.suronc.2022.101772.
- Addeo P, Locicero A, Bachellier P. Circumportal pancreas. J Visc Surg. 2019 Oct;156(5):467-468. doi: 10.1016/j.jviscsurg.2019.02.001.
- Yamaguchi K, Sato N, Minagawa N, Matsumura M, Mori Y, Tamura T, Shibao K, Higure A. Sarcoidosis in a patient with a circumportal pancreas with a retroportal main pancreatic duct: a case report. 2013 Oct;42(7):1197-9. doi: 10.1097/MPA.0b013e31827e2d20.
- Pandrowala S, Parray A, Chaudhari V, Shrikhande SV, Bhandare MS. Portal Annular Pancreas (PAP): an Underestimated Devil in Pancreatic Surgery-Systematic Review of Literature and Case Report. J Gastrointest Surg. 2021 May;25(5):1332-1339. doi: 10.1007/s11605-021-04927-0.