Despoina Avramidou, Vasileios Papadopoulos
Department of Internal Medicine, Xanthi General Hospital, Xanthi, Greece
Correspondence Address: Vasileios Papadopoulos, MD, PhD, 2, Staliou str – 67132 Xanthi, Greece, email: vaspapmd@gmail.com
Intracerebral hemorrhage (ICH) is a major public health concern leading to high rate of mortality as well as disability [1]. Many risk factors for ICH have been described including old age, male sex, arterial hypertension, diabetes mellitus, and high alcohol intake [2, 3]. Moreover, anticoagulants and antiplatelets have also been documented to increase the risk for ICH [4].
The role of platelets in the pathophysiology of (ICH) has not been clarified yet. It has been demonstrated that platelet count (PLT) was significantly lower in hemorrhagic strokes when compared with controls [5]. Moreover, PLT has been proposed as an independent predictor of poor outcome at time of discharge in cerebellar hemorrhage [6]. Interestingly, the presence of thrombocytopenia (PLT < 150 x 103/μL) itself did not affect the functional outcome after ICH, regardless of antiplatelets administration [7]. However, whether PLT can be a prognosticator of survival after ICH remains obscure.
We have retrospectively enrolled 60 patients, aged 75.9 ± 12.0 years, who had been admitted to Xanthi General Hospital for ICH between January 2018 and May 2021. Demographics, medical record, vital signs, arterial blood gas test, complete blood count, blood biochemistry, and CT scan test were collected for each patient. PLT at admission (PLT1) and 24 hours later (PLT2) were evaluated as potential predictors of 30-day survival after ICH. Descriptive statistics are provided either as means along with their relevant standard deviations, or percentages, for scale and nominal variables respectively. Survival data are given as medians along with their 25th and 75th percentiles (Q1 and Q3, respectively). All patients were censored, either at the time of their death, or at the 31st day after admission. Kaplan Meier (KM) curves were used to depict survival data; the log-rank test was used to determine the univariate significance of the study variables. Cox proportional hazards regression analysis was performed to explore the potential correlations between independent variables and survival data. All reported p values are two-sided. The level of statistical significance was set to p=0.05. All numerical values are given with at least two significant digits. Statistical analysis was performed with the use of IBM SPSS Statistics software, version 26.0, for Windows. MedCalc software, version 20.218, was preferred for visualization of results. The study was approved by the Scientific Board of Xanthi General Hospital (Decision No. 103/May 17, 2021).
Thirty patients (50%) succumbed within the first 30 days. The median survival time during the first month after admission was 25 days (Q1: 11 days; Q3: 30 days).
PLT1 values were 232,000 ± 86,000/μL and 211,000 ± 60,000/μL (P=0.251), while PLT2 values were 240,000 ± 63,000/μL and 203,000 ± 60,000/μL (P=0.012), for survivors and non-survivors, respectively; these results are schematically presented as boxplots. (Figure 1)
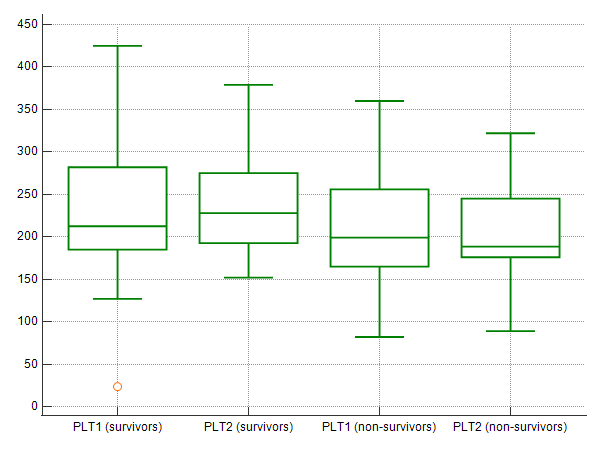
Figure 1. Boxplots presenting PLT1 and PLT2 values in survivors and non-survivors; PLT values (y-axis) are given in 103/μL.
Using Cox proportional hazards regression univariate analysis, it has been shown that 30-day survival after ICH was positively correlated with PLT2 (P=0.012), hemoglobin levels at admission (P=0.020), and oxygen saturation at admission (P=0.015). Moreover, 30-day survival was negatively correlated with age (P=0.001), blood glucose levels at admission (P=0.043), medical history of diabetes mellitus (P=0.014), and medical history of arterial hypertension (P=0.036). Of note, PLT1 was comparable between survivors and non-survivors (P=0.251).
The use of a Cox-regression proportional hazards multivariate analysis model demonstrated that increased PLT2 was independently correlated with 30-day survival after ICH, considering all other parameters as potential confounders (HR: 0.986 per unit 103/μL; 95% CI: 0.978-0.994, P<0.001). All necessary details are provided in Table 1.
Table 1. Patients’ characteristics as well as Cox regression univariate and multivariate analysis based on 30-day survival status.
Parameters | Mean±SD; N(%)† | Survived (n=30) | Succumbed (n=30) | P‡ | HR; ±95%CI§ | P§ |
Gender |
|
|
|
|
|
|
Men | 28 (46.7) | 14 (46.7) | 14 (46.7) |
|
|
|
Women | 32 (53.3) | 16 (53.3) | 16 (53.3) | 0.834 |
|
|
Age (years) |
|
|
|
|
|
|
Mean ± SD | 75.9 ± 12.0 | 70.8 ± 11.2 | 81.0 ± 10.5 | 0.001 | 1.035; 0.992-1.080 | 0.115 |
Diabetes |
|
|
|
|
|
|
Yes | 10 (16.7) | 1 (3.3) | 9 (30.0) |
| 1.000 |
|
No | 50 (83.3) | 29 (96.7) | 21 (70.0) | 0.014 | 0.651; 0.216-1.960 | 0.445 |
Hypertension |
|
|
|
|
|
|
Yes | 42 (70.0) | 17 (56.7) | 25 (83.3) |
| 1.000 |
|
No | 18 (30.0) | 13 (43.3) | 5 (16.7) | 0.036 | 0.183; 0.055-0.614 | 0.006 |
Antiplatelets |
|
|
|
|
|
|
Yes | 14 (23.3) | 7 (23.3) | 7 (23.3) |
|
|
|
No | 46 (76.7) | 23 (76.7) | 23 (76.7) | 0.949 |
|
|
Anticoagulants |
|
|
|
|
|
|
Yes | 7 (11.7) | 3 (10.0) | 4 (13.3) |
|
|
|
No | 53 (88.3) | 27 (90.0) | 26 (86.7) | 0.995 |
|
|
Temperature (oC) |
|
|
|
|
|
|
Mean ± SD | 36.2 ± 0.4 | 36.1 ± 0.3 | 36.2 ± 0.5 | 0.186 |
|
|
Pulse rate (min-1) |
|
|
|
|
|
|
Mean ± SD | 86.8 ± 16.5 | 84.8 ± 17.2 | 88.7 ± 15.9 | 0.451 |
|
|
SBP (mmHg) |
|
|
|
|
|
|
Mean ± SD | 170 ± 29 | 173 ± 31 | 168 ± 28 | 0.565 |
|
|
DBP (mmHg) |
|
|
|
|
|
|
Mean ± SD | 94.2 ± 14.3 | 94.7 ± 15.4 | 93.7 ± 13.4 | 0.611 |
|
|
Hemoglobin (g/dL) |
|
|
|
|
|
|
Mean ± SD | 13.3 ± 1.4 | 13.7 ± 1.1 | 12.9 ± 1.5 | 0.020 | 0.554; 0.401-0.766 | <0.001 |
Glucose (mg/dL) |
|
|
|
|
|
|
Mean ± SD | 124 ± 46 | 111 ± 25 | 138 ± 57 | 0.043 | 1.000; 0.989-1.011 | 0.994 |
Creatinine (mg/dL) |
|
|
|
|
|
|
Mean ± SD | 0.97 ± 0.91 | 1.03 ± 1.15 | 0.90 ± 0.60 | 0.667 |
|
|
CRP (mg/dL) |
|
|
|
|
|
|
Mean ± SD | 1.45 ± 1.53 | 1.13 ± 1.53 | 1.77 ± 1.49 | 0.206 |
|
|
pH |
|
|
|
|
|
|
Mean ± SD | 7.40 ± 0.07 | 7.41 ± 0.06 | 7.40 ± 0.08 | 0.717 |
|
|
sPO2 (%) |
|
|
|
|
|
|
Mean ± SD | 95.0 ± 2.9 | 95.8 ± 1.5 | 94.2 ± 3.6 | 0.015 | 0.854; 0.750-0.973 | 0.018 |
PLT1 (103/μL) |
|
|
|
|
|
|
Mean ± SD | 222 ± 74 | 232 ± 86 | 211 ± 60 | 0.251 |
|
|
PLT2 (103/μL) |
|
|
|
|
|
|
Mean ± SD | 223 ± 64 | 240 ± 63 | 203 ± 60 | 0.012 | 0.986; 0.978-0.994 | <0.001 |
SD: Standard Deviation, † For scale and nominal variables, respectively,
‡ P-value based on univariate Cox regression analysis,
- HR along with ±95% CI and P-value based on multivariate Cox regression analysis
To further assess an easy and clinically useful tool for using PLT2 as a predictor of 30-day survival, we used 225,000/μL, being the mean of the PLT2 covariate, as derived from the Cox proportional hazards regression univariate analysis. Patients with PLT2 ≥225,000/μL had a hazard ratio (HR) of 0.991 (95% CI: 0.984-0.998) per unit (103/μL) to succumb within the first 30 days after admission for ICH (HR<1 favors 30-day survival). The result was statistically significant (P=0.048) using the Log-Rank test; the relevant Kaplan-Meier curve is provided as Figure 2.
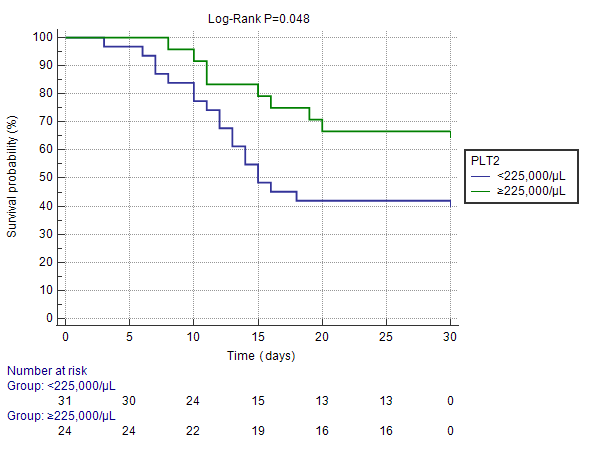
Figure 2. Kaplan-Meier curve depicting survival function according to selected PLT2 cutoff; ICH patients with PLT2 ≥225,000/μL presented favorable 30-day survival when compared to patients with PLT2 <225,000/μL (Log-Rank P=0.048).
Based on our results, we propose that PLT2 might be further investigated as an early predictor of 30-day survival after ICH. Moreover, in the light of absence of independent correlation between PLT1 and 30-day survival, it is reasonable to hypothesize that the crucial parameter of platelet involvement in ICH might not be their initial number per se, but rather their alterations due to vascular damage and/or activation.
References
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009 May 9;373(9675):1632-44. doi: 10.1016/S0140-6736(09)60371-8. PMID: 19427958; PMCID: PMC3138486.
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003 Aug;34(8):2060-5. doi: 10.1161/01.STR.0000080678.09344.8D. Epub 2003 Jul 3. PMID: 12843354.
- Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010 Jun 26;375(9733):2215-22. doi: 10.1016/S0140-6736(10)60484-9. Erratum in: Lancet. 2010 Sep 18;376(9745):958. Hillage, H L [corrected to Hillege, H L]. PMID: 20609967; PMCID: PMC2904878.
- Zeng Z, Chen J, Qian J, Ma F, Lv M, Zhang J. Risk Factors for Anticoagulant-Associated Intracranial Hemorrhage: A Systematic Review and Meta-analysis. Neurocrit Care. 2023 Jan 20. doi: 10.1007/s12028-022-01671-4. Epub ahead of print. PMID: 36670269.
- Sadeghi F, Kovács S, Zsóri KS, Csiki Z, Bereczky Z, Shemirani AH. Platelet count and mean volume in acute stroke: a systematic review and meta-analysis. Platelets. 2020 Aug 17;31(6):731-739. doi: 10.1080/09537104.2019.1680826. Epub 2019 Oct 26. PMID: 31657263.
- Lin CY, Chang CY, Sun CH, Li TY, Chen LC, Chang ST, Wu YT. Platelet count and early outcome in patients with spontaneous cerebellar hemorrhage: a retrospective study. PLoS One. 2015 Mar 17;10(3):e0119109. doi: 10.1371/journal.pone.0119109. PMID: 25781880; PMCID: PMC4364557.
- Mrochen A, Sprügel MI, Gerner ST, Sembill JA, Lang S, Lücking H, Kuramatsu JB, Huttner HB. Thrombocytopenia and Clinical Outcomes in Intracerebral Hemorrhage: A Retrospective Multicenter Cohort Study. Stroke. 2021 Jan;52(2):611-619. doi: 10.1161/STROKEAHA.120.031478. Epub 2021 Jan 12. PMID: 33430632.
